
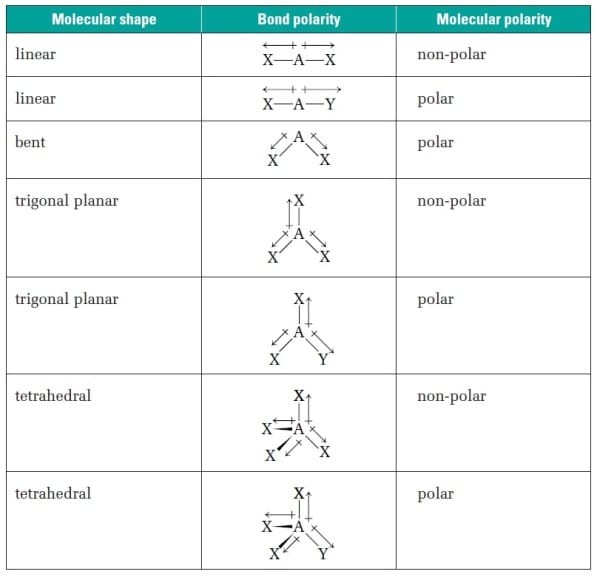
When drawing the Lewis structure of water, oxygen is the central atom. Double and triple bonds still count as 1 bonding domain (BD)!Įxample: H 2O.So count only the # pairs around the central atom not in the entire compound! NOTE: the # of BD and NBD is from the perspective of the CENTRAL ATOM.Now within that arrangement, what is the specific combination and geometry.Count the TOTAL number of electron domains (both bonding and non-bonding) and classify the ARRANGMENT.Draw the Lewis structure of the molecule.Each particular combination will determine a geometry.ĭetermining Electron Arrangement/Geometry Rules: Then we look to see exactly how the total electron domains are distributed-meaning how many are bonding and how many are non-bonding (or lone pairs). The total count will determine the arrangement.

Electron domains are pairs of electrons so we count both bonding pairs and non-bonding pairs to get the TOTAL. We count the TOTAL # of electron domains. Arrangement is the general classification of “shape” but geometry is the specific “shape” of the molecule in question.įor example: 3 sided figures: triangles (general shape) BUT 3 sided figure with equal sides: equilateral triangle (a specific kind of triangle). Note: The difference between an arrangement and geometry is as follows. It allows us to predict the shape of a molecule 3-dimensionally. VSEPR: Valence Shell Electron Pair Repulsion model uses the repulsion of electron pairs to determine strain on various bonds and the resulting electron ARRANGEMENT and GEOMETRY.


 0 kommentar(er)
0 kommentar(er)
